
Maternal dengue infections decrease birth weight, increase the risk for low birth weight and the number of hospitalisations of children in the first three years of life
A number of infections during pregnancy, including rubella (Dudgeon 1967), malaria (Barecca 2010), and influenza (Kelly 2011) have been shown to affect the development of unborn children. These lead to worse health at birth which has long-lasting consequences, ranging from reduced educational attainment, worse labour market outcomes and worse health in later life (Black et al. 2007). Recently, the Zika virus has been added to the list of infections identified as a risk factor for congenital conditions, most prominently causing microcephaly in Brazil (Lowe 2018, Lautharte and Rasul 2020). Dengue, a virus related to Zika and endemic in Brazil, has previously not been considered to pose a significant threat to unborn children, leading to the omission of dengue from the TORCH list of infections (Jaan and Rajnik 2022). This is despite dengue fever being by far the most prevalent mosquito-borne disease globally, exposing half of the world’s population to the risk of infection. Previous research on maternal dengue focused on small samples of women hospitalised with dengue or rare severe dengue infections, providing an inconclusive picture of the effect of maternal dengue infections on new-borns’ health.
Maternal dengue and birth outcomes: Comparing multiple births of the same mother
In a recent paper (Foureaux Koppensteiner and Menezes 2023), we provide novel evidence on the negative health consequences of maternal dengue infections using large linked administrative data sets from Brazil. In a departure from previous work, this paper focuses on the vast majority of mild and moderate dengue infections previously considered unproblematic during pregnancy. To provide causal estimates, we focus on mothers with multiple births, enabling us to estimate maternal-fixed effects specifications. We essentially compare the outcomes for pregnancies with and without maternal dengue infections for the same mother and control for neighbourhood and time-varying mother and pregnancy characteristics. Contrary to previous studies, we document a significant negative effect of even mild and moderate dengue infections on a range of birth outcomes.
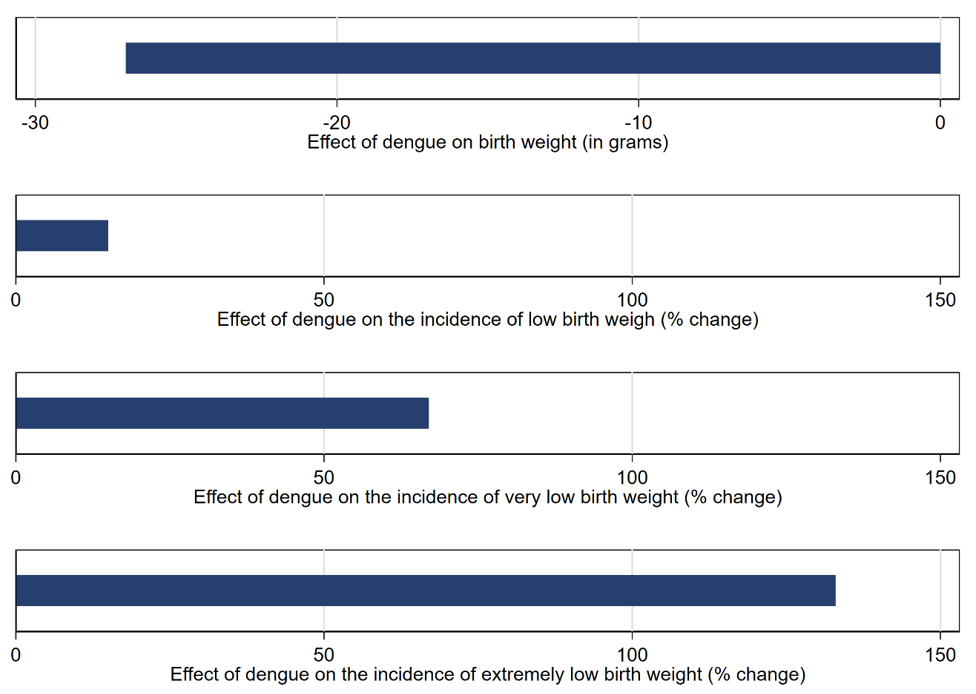
We find that dengue during gestation reduces birth weight of children by about 27 grams on average (Figure 1). The magnitude of the estimated effect is similar in size to the effects of maternal influenza (Schwandt 2017), or the (positive) effect of a generous cash transfer in Uruguay (Amarante 2016). The average effects on birth weight nevertheless conceal much more pronounced effects at lower parts of the birth weight distribution. We find that maternal dengue increases the risk for new-borns being classified as very and extremely low birth weight by 67% and 133% compared to the baseline incidence, respectively. The effects on birth weight are matched with a similar increase in the risk for very preterm birth (before week 32) of 77%. Low birth weight has been shown to negatively affect socio-economic outcomes and health in adulthood (Oreopoulos et al. 2008, Almond and Currie 2011), and our results indicate that maternal dengue contributes to the list of factors during pregnancy with long-lasting consequences for human capital.
Figure 1: Effect of dengue on birth outcomes (Foureaux Koppensteiner and Menezes 2023)
Persistent effects on health
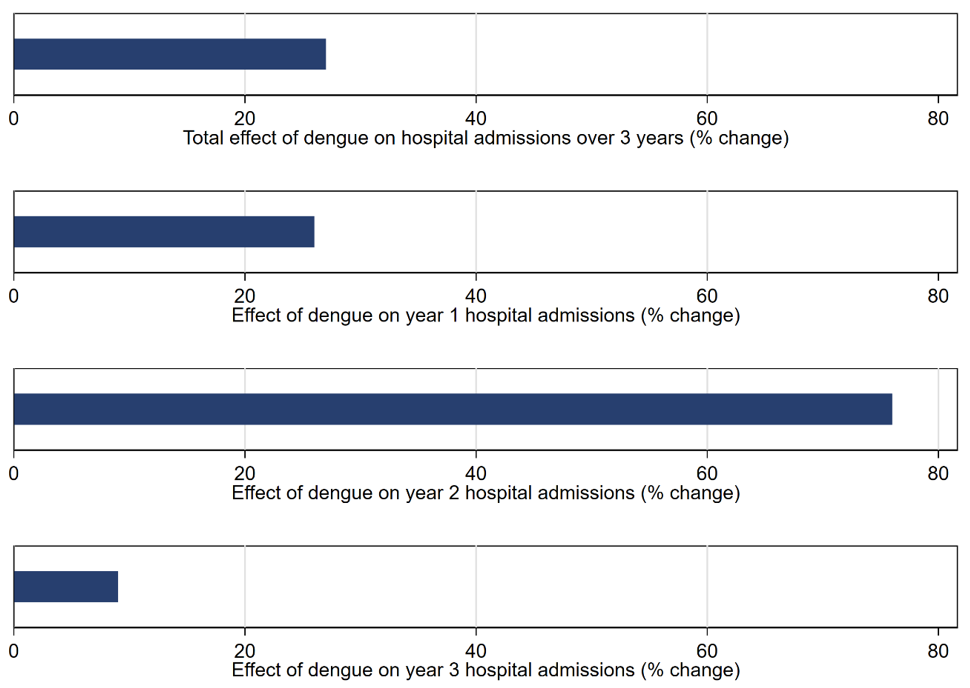
To investigate the persistence of the negative consequences of maternal dengue, we further link birth records with hospitalisation records. We find that maternal dengue during pregnancy increases the risk of hospitalisation of children over a three-year period after birth substantially by 27% compared to the mean incidence (Figure 2). We also find that the effects are long-lasting, with the strongest relative effect sizes estimated in the second year after birth, leading to a 76% increase in hospitalisations. Using information on the costs of hospital treatment for each admission, we find that maternal dengue substantially increases subsequent medical expenditures from hospitalisation in the first and second year after birth. The increase in hospital admissions points to the negative longer-term health consequences of maternal dengue and also to the previously neglected cost of maternal dengue infections on public spending and utilisation of scarce public health resources.
Figure 2: Effect of dengue on the hospitalisation of children (Foureaux Koppensteiner and Menezes 2023)
Implications for public health policy
Due to the prevalence of the dengue virus in over 120 countries, these new results are concerning, affecting the health and long-term development of millions of children born every year. As countries around the tropics—already disadvantaged with generally worse provision of health and antenatal services— suffer from higher dengue incidence, maternal dengue infections add to the burden on health services and contribute to the intergenerational transmission of poverty. With the expansion of the dengue vector due to climate change further away from the tropics, the number of pregnant mothers exposed to dengue will likely increase further in the next decades.
The results are relevant for a range of public health policies, ranging from vector control and updated risk communication with key target groups to vaccine adoption and prioritisation of recipient groups. Updated cost estimates of the consequences of dengue infections, in particular from directly identifiable costs due to hospitalisation of children, are important inputs in cost-benefit analysis of vector control programmes of aedes aegypti—the mosquito responsible for transmitting dengue, Zika and chikungunya virus—and for programmes to publicly provide insect repellent to expectant mothers on low-income during the Zika epidemic (Goldman 2017). Similarly, other policies aimed at protecting individuals, especially pregnant women and women and girls of reproductive age, through public awareness campaigns have been proposed in response to Zika by the WHO (2016) and should be considered in response to the dengue virus in line with advice on other TORCH infections. Lastly, a new generation of dengue vaccine, effective against all four strains of dengue, has recently been approved by the Brazilian regulator ANVISA and in other countries, and the findings on the grave consequences of maternal dengue may aid the decisions regarding suitability and prioritisation of the vaccines.
References
Almond, D and J Currie (2011), “Killing Me Softly: The Fetal Origins Hypothesis,” Journal of Economic Perspectives, 25(3), 153-172.
Barreca, A (2010), “The Long-term Economic Impact of in Utero and Postnatal Exposure to Malaria,” Journal of Human Resources, 45(4), 865-892.
Dudgeon, J A (1967), “Maternal Rubella and its Effect on the Foetus,” Archives of Disease in Childhood, 42(224), 110-125.
Foureaux Koppensteiner, M and L Menezes (2023), “Maternal Dengue and Health Outcomes of Children,” American Economic Journal: Applied Economics, conditionally accepted.
Goldman, H (2017), “Insect Repellants during Pregnancy in the Era of the Zika Virus,” Obstetrics and Gynecology, 128(5), 1111-1115.
Kelly, E (2011), “The scourge of Asian flu: In utero Exposure to Pandemic Influenza and the Development of a Cohort of British Children,” Journal of Human Resources, 46(4), 669-694.
Lowe, R, C Barcellos, P Brasil, OG Cruz, NA Honório, H Kuper, MS Carvalho (2018), “The Zika Virus Epidemic in Brazil: From Discovery to Future Implications,” International Journal of Environmental Research and Public Health, 15(1), 96.
Lautharte, I and I Rasul (2020), “The Anatomy of a Public Health Crisis: Household responses during the Zika epidemic in Brazil,” VoxDev, 29.05.2020.
Oreopoulos, P, M Stabile, R Walld, and LL Roos (2008), “Short-, Medium-, and Long-Term Consequences of Poor Infant Health,” Journal of Human Resources, 43(1), 88-138.
Jaan A, and M Rajnik (2022), “TORCH Complex,” In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560528/.
WHO (2016), “Zika Strategic Response Framework,” Quarterly update from 25 October 2016, WHO/ZIKV/SRF/16.4. Available online: https://www.who.int/publications/i/item/zika-strategic-response-plan.


