
The Green Revolution dramatically altered the agricultural and dietary landscape in India. While calorie availability increased, reduced dietary diversity had adverse long-term health impacts, meaning the overall impact is only now being understood.
Over the past half-century, the Green Revolution has profoundly boosted agricultural productivity, alleviating concerns about food insecurity (Evenson and Gollin 2003). Recent work by Gollin et al (2021, featured on VoxDev) documents a resulting increase in GDP and living standards in the developing world, while other work studies the risk of prioritising the agricultural sector at the expense of manufacturing growth (Moscona 2019, featured on VoxDev).
The impact of the Green Revolution on health is similarly complex, theoretically. Growth in agricultural output can increase calorie intake, reducing under-nutrition and improving infant mortality (Bharadwaj et al. 2020, von der Goltz 2020), but the growth in calorie consumption can also contribute to obesity and chronic disease, especially if, as in the case of the Green Revolution in India, the increase in food production is concentrated in carbohydrate-heavy rice and wheat. Chronic diseases, such as diabetes, are leading causes of mortality in the developing world (WHO 2016), despite being previously considered “rich country diseases.” Many countries currently struggle with a double burden of malnutrition – high rates of under-nutrition and rising rates of obesity – and experts suggest that dietary changes are one contributing factor (Popkin 2001).
In our research (Sekhri and Shastry, forthcoming), we show that the agricultural productivity growth spurred by the Green Revolution also contributed, unexpectedly, to the rise in chronic disease. In doing so, we highlight the importance of dietary diversity, traditionally a subordinate goal of nutrition policy in developing countries.
Geographic variation in adoption of high-yield varieties
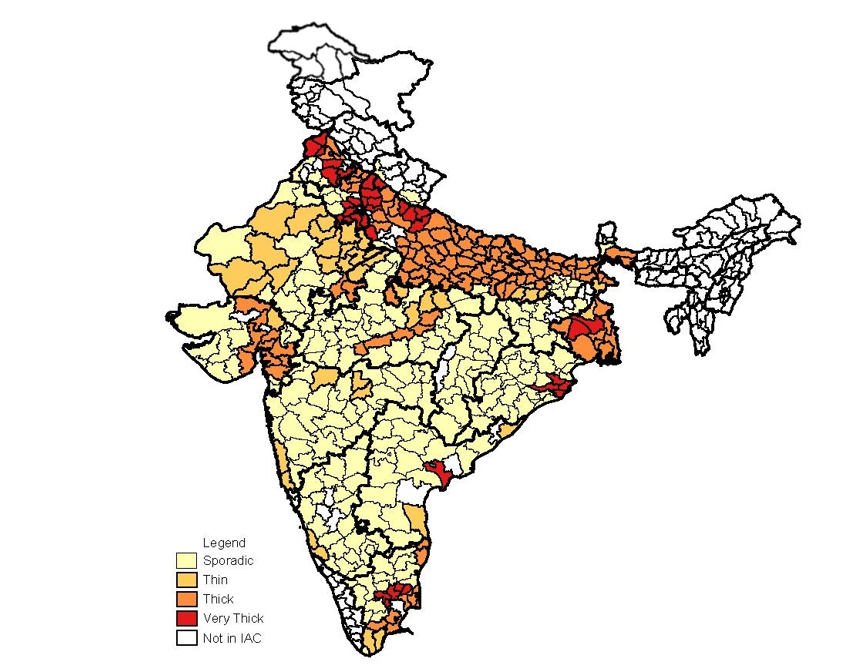
The Green Revolution arrived in India in 1966, when the government introduced high-yield varieties of rice and wheat. As predicted by Norman Borlaug, these high-yield varieties were most successful in well-irrigated land (Saha 2013). Timely irrigation was essential, leading to greater adoption of high-yield varieties in districts with abundant underground aquifers (Sengupta and Ghosh 1968). Figure 1 graphs the average area planted with high-yield varieties over time in groundwater-rich districts and groundwater-scarce districts, separately. Figure 2 provides a map of Indian districts in the India Agricultural and Climate Dataset, shaded by aquifer thickness (IAC, Matlon 1999). While high-yield varieties were adopted across all types of districts, these two figures demonstrate substantial geographic variation in exposure to the Green Revolution.
Figure 1: Adoption of high-yield varieties in districts with different groundwater availability
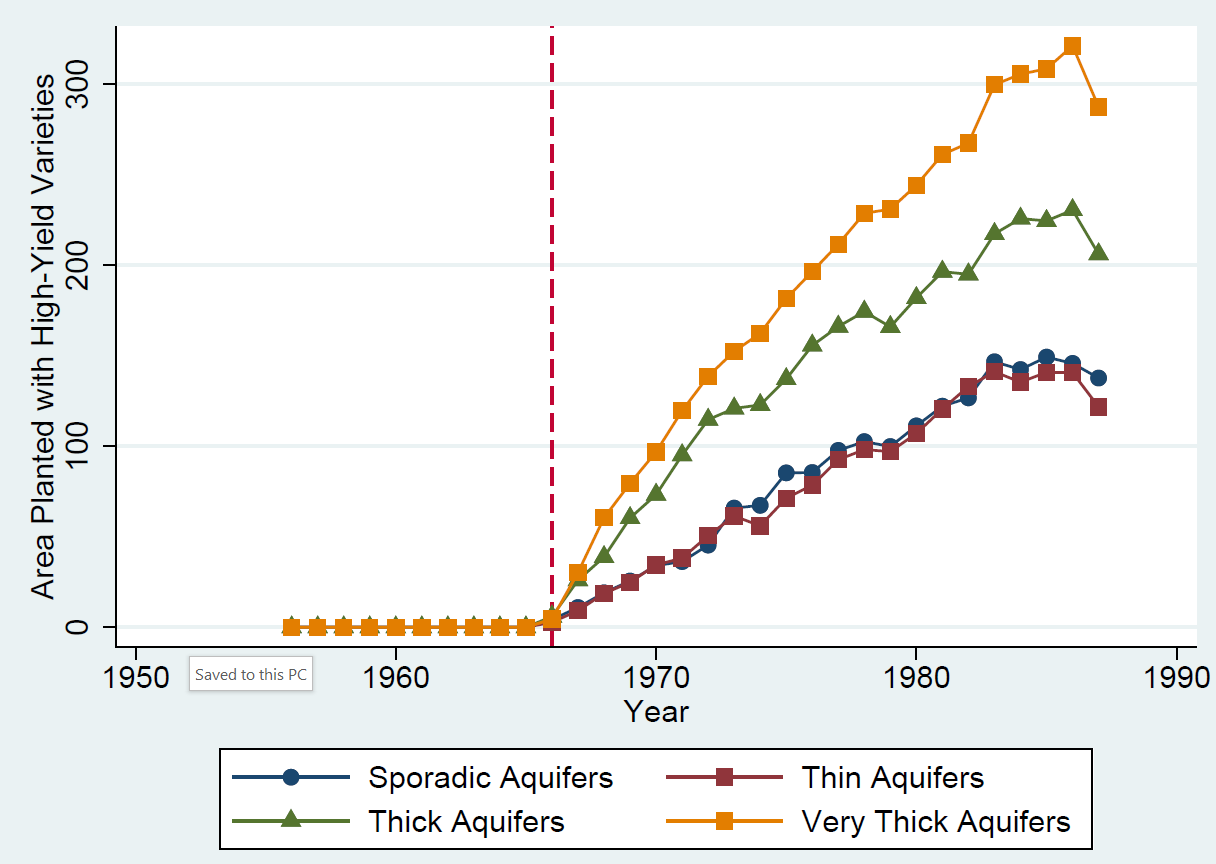
Figure 2: Map of Indian districts in the IAC, shaded by aquifer thickness
How did the Green Revolution impact rates of chronic disease?
To identify the causal effect of the Green Revolution on rates of chronic disease, we use both the geographic variation and temporal variation (birth year relative to the start of the Green Revolution) in exposure. Simply comparing differences in rates of chronic disease across districts with greater or lesser exposure to the Green Revolution would risk conflating the effect of the Green Revolution with any underlying differences between districts. Similarly, comparing people born before and after the Green Revolution would risk conflating the effect of the Green Revolution with other changes over time. Our strategy avoids both risks, by comparing differences in rates of chronic disease among individuals born before and after 1966 in districts with substantial exposure to the Green Revolution to the same difference in districts with limited exposure. This strategy pinpoints the effect of exposure to the Green Revolution at very young ages under the assumption that trends in chronic disease would have been similar across districts had the Green Revolution not occurred. We find support for this assumption.
Using data from the 2005 India Human Development Survey (IHDS, Desai et al. 2005), we find that men exposed to the Green Revolution in early childhood (in utero and maybe in the first few years of life) were more likely to report a diabetes diagnosis. Figure 3 shows this increase in diabetes prevalence for men with greater exposure to the Green Revolution in their early childhood.
Figure 3: Impact of exposure to the Green Revolution on diabetes prevalence
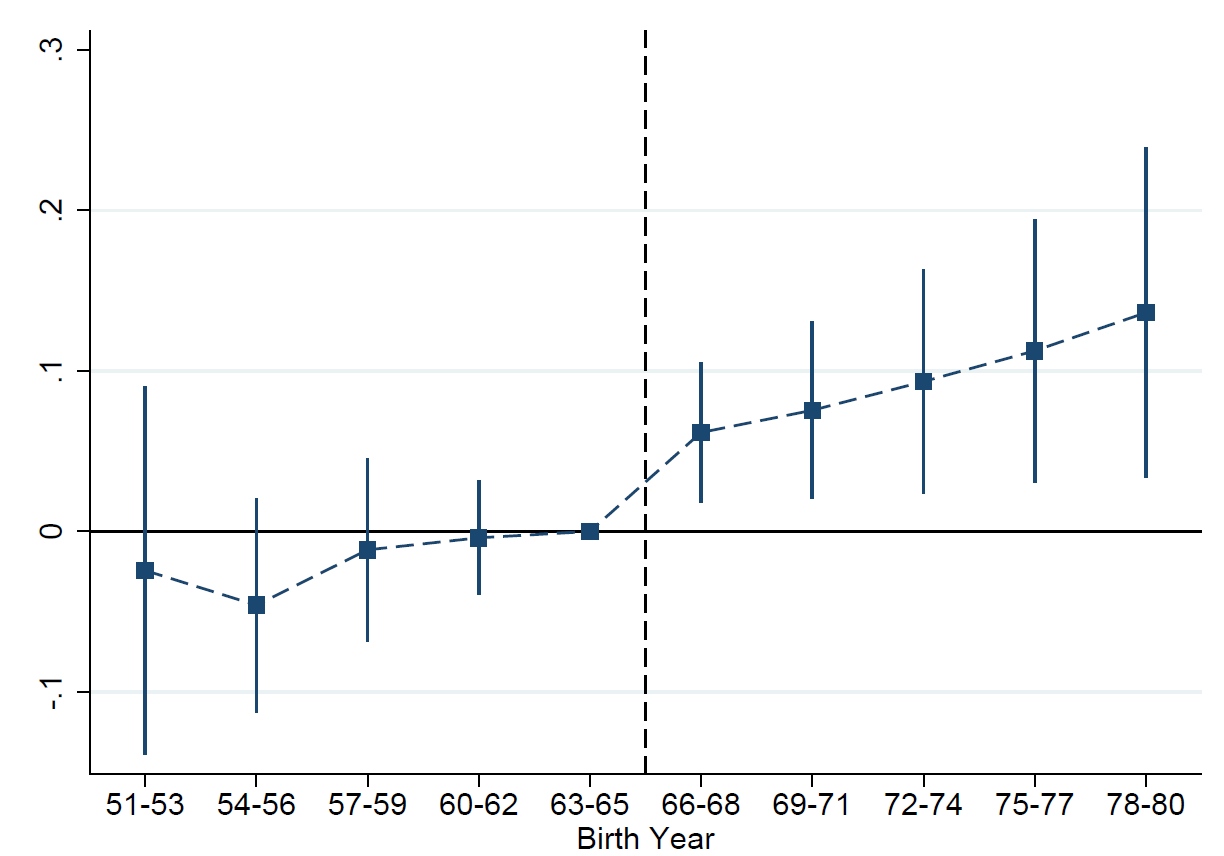
The oldest men exposed to the Green Revolution in early childhood are only 38-39 years old in the IHDS; many men with diabetes may have not yet been diagnosed. We find corroborating evidence of an increase in diabetes using a second household survey, the 2015-16 National Family Health Survey (NFHS, NFHS-4, 2017b), that collects biomarkers, including glucose levels. We estimate an increase in diabetes prevalence of approximately 4 percentage points, on a base of 26% among men older than 49.
Interestingly, we do not find any significant increase in chronic disease for women, possibly because the available data (IHDS but not NFHS) were collected too early to see an effect for women (the oldest affected cohort being 38-39) who are at higher risk of metabolism-related chronic disease after menopause.
A possible mechanism: In utero and early childhood nutrition
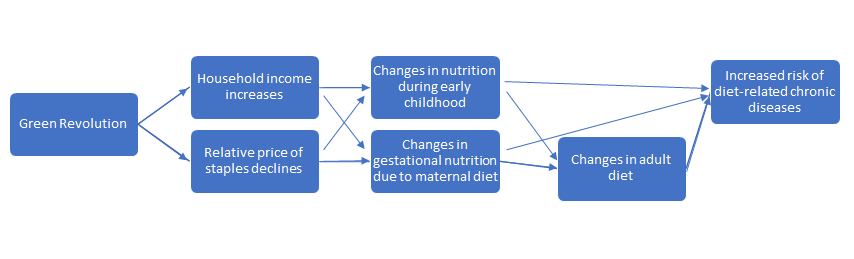
Our strategy estimates the effect of the Green Revolution at very young ages. Those born before the Green Revolution, our control group, would also be affected by the availability of high-yield crops, just at older ages. Looking at Figure 3, however, diabetes prevalence among cohorts born before 1966 appears unrelated to age at first exposure, suggesting that the effect occurs during gestation and early childhood. We provide suggestive evidence that the increase in diabetes is due to changes in nutrient composition at these ages and offer evidence against other possible mechanisms. Figure 4 demonstrates our theory of change.
Figure 4: Theory of change: How exposure to the Green Revolution in early childhood affects diabetes risk
Motivated by the Barker hypothesis that in utero nutrition can have long-lasting impacts on health (Barker 1990), a large literature in economics has documented a beneficial impact of improved nutrition in utero and early childhood on long term health (see reviews in Almond and Currie 2011 and Almond et al. 2018), including chronic diet-related diseases (Hoynes et al 2016). Our results run counter to these findings since food was more plentiful while in utero for those born after the Green Revolution. We hypothesise that changes in the composition of maternal diet are responsible. While studies in humans are lacking, a low-protein maternal diet has been found to increase glucose intolerance in rats and mice (Dahri et al. 1991; Nivoit et al. 2009; Samuelsson et al. 2008; Stocker et al. 2005).
We provide evidence supporting this hypothesis. First, while we do not have data on dietary intake for pregnant women in the 1960s, agricultural data indicates that the production of pulses, a primary source of protein in India, fell in groundwater-rich districts as more agricultural land was allocated to staples. Second, the impact on diabetes is driven by households whose primary staple is rice not wheat: relative to wheat, rice has half the amount of protein, more carbohydrates, and a higher glycemic index (Foster-Powell et al. 2002). Our hypothesis is consistent with other evidence of nuanced impacts of the Green Revolution on nutrition: Headey and Hoddinott (2016) show that the Green Revolution in Bangladesh improved child weight-for-height but not height-for-age.
Finally, we rule out alternative mechanisms. Our results are robust to other factors contributing to metabolic disease in India, such as trends in lifestyle, because those should affect men born before the Green Revolution just as much as those born after. We also find no evidence of an increase in other, non-diet-related chronic diseases such as cancer, asthma, and tuberculosis, suggesting that other changes to the early childhood environment such as the use of fertiliser are not driving our results.
Conclusion and implication for policymakers
Our study shows that the health effects of the Green Revolution are more nuanced and long-lasting than previously understood. The increase in calorie availability contributed to reductions in infant mortality, but changes in the composition of those calories contributed to the rise in diabetes. Comparing these effects requires making a number of assumptions on survival to adulthood and the impact of diabetes on life expectancy - a back-of-the-envelope calculation suggests that the years of life lost due to the increase in diabetes are of a similar magnitude to the years of life gained due to the reduction in infant mortality.
These findings have implications for agricultural policy and welfare policy. Nutrition policy in the developing world has heretofore focused on calorie consumption and under-nutrition. We highlight the importance of dietary diversity. To take one example, the Public Distribution System in India should encourage protein consumption; it currently promotes, inadvertently, a low-protein, high-calorie diet by prioritising the distribution of wheat, rice, and sugar.
References
Barker, D J (1990), “The fetal and infant origins of adult disease,” BMJ: British Medical Journal 301(6761): 1111.
Bharadwaj, P, J Fenske, N Kala, and R A Mirza (2020), “The Green Revolution and infant mortality in India,” Journal of Health Economics 71: 102–314.
Dahri, S, A Snoeck, B Reusens-Billen, C Remacle, and J J Hote (1991), “Islet function in offspring of mothers on low-protein diet during gestation,” Diabetes 40 (Supplement 2): 115–120.
Desai, S, R Vanneman, and National Council of Applied Economic Research (2005), India Human Development Survey (IHDS), 2005. Interuniversity Consortium for Political and Social Research [distributor], 2018-08-08. https://doi.org/10.3886/ICPSR22626.v12.
Evenson, R E and D Gollin (2003), “Assessing the impact of the Green Revolution, 1960 to 2000,” Science 300(5620): 758-762.
Foster-Powell, K, S H Holt, and J C Brand-Miller (2002), “International table of glycemic index and glycemic load values: 2002,” The American Journal of Clinical Nutrition 76(1): 5–56.
Gollin, D, C W Hansen, and A M Wingender (2021), “Two blades of grass: The impact of the Green Revolution,” Journal of Political Economy 129(8): 2344–2384.
Headey, D D and J Hoddinott (2016), “Agriculture, nutrition and the green revolution in Bangladesh,” Agricultural Systems 149: 122–131.
Hoynes, H, D W Schanzenbach, and D Almond (2016), “Long-run impacts of childhood access to the safety net,” American Economic Review 106(4): 903–34.
Matlon, P (1999), Measuring the impact of climate change on Indian agriculture. (World Bank Technical Paper No. 402). Edited by A. Dinar, R. Medelsohn, R. Evenson, J. Parikh, A. Sanghi, K. Kumar, J. McKinsey and S. Lonergan. Washington DC: The World Bank (1998), pp. 266, US 20.00.ISSN0253 − 7494. Experimental Agriculture 35(4), 507−516.
Moscona, J (2019). “Agricultural Development and Structural Change, Within and Across Countries,” Working paper, Harvard University.
NFHS-4 (2017b), National Family Health Survey (NFHS-4), 2015-16: India. International Institute for Population Sciences (IIPS) and ICF, Mumbai:IIPS.
Nivoit, P, C Morens, F Van Assche, E Jansen, L Poston, C Remacle, and B Reusens (2009), “Established diet-induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance,” Diabetologia 52(6): 1133–1142.
Popkin, B M (2001), “The nutrition transition and obesity in the developing world,” The Journal of Nutrition 131(3): 871S–873S.
Saha, M (2013), “Food for soil, food for people: Research on food crops, fertilizers, and the making of ‘modern’ Indian agriculture,” Technology and Culture 54(2): 289–316.
Samuelsson, A-M, P A Matthews, M Argenton, M R Christie, J M McConnell, E H Jansen, A H Piersma, S E Ozanne, D F Twinn, C Remacle, et al. (2008), “Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hyper-tension, and insulin resistance: a novel murine model of developmental programming,” Hypertension 51(2), 383–392.
Sekhri, S and G K Shastry (2024), “The Curse of Plenty: The Green Revolution and the Rise in Chronic Disease,” American Economic Journal: Applied Economics, forthcoming.
Sengupta, S and M Ghosh (1968), “HYVP for rice: Performance in a Bengal district,” Economic and Political Weekly, A26–A28.
Stocker, C J, J R Arch, and M A Cawthorne (2005), “Fetal origins of insulin resistance and obesity,” Proceedings of the Nutrition Society 64(2): 143–151.
von der Goltz, J, A Dar, R Fishman, N D Mueller, P Barnwal, and G C McCord (2020). “Health impacts of the Green Revolution: Evidence from 600,000 births across the developing world,” Journal of Health Economics 74, 102373.
WHO (2016). Global Report on Diabetes. World Health Organization


